We have completed joint research with Shoken Techno Co., Ltd. and Techno Solute Lab Co., Ltd. as well as with Saga University and Osaka Prefectural University.
Purpose
The aim is to create products that efficiently remove heavy metals from water and seawater.
Result
World-class performance for cesium and lead.
Strontium and cadmium are standard performance.
Unfortunately, arsenic does not adsorb.
Features
The materials are generally available and no chemicals are used.
It maintains a performance of about 80% even in seawater.
The shape of the ceramic ball is easy to use.
It keeps its shape in the water.
Once adsorbed metal ions are not re-released even if the adsorbent is pulverized.
As it does not become sludge like zeolite, final disposal is easy.
The price is low.
Main Applications
cesium
strontium
nuclear related
cadmium
water quality improvement
lead
battery recycling facility
softening of hard water
Technical Report on Characteristics and Adsorption Properties of Adsorbent Black Holes
These are excerpts from the main points.
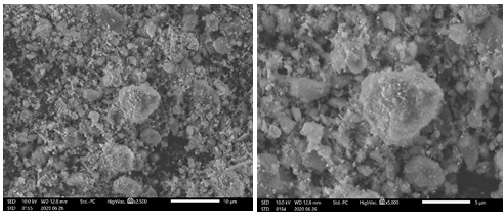
4)Scanning Electron Micrograph Organization Observation
Fig. 4 shows the SEM image of the fracture surface of BH.
5.Adsorption experiment of BH and its results

1)Adsorption experiment
In the experiment, a predetermined solution was placed in a beaker, the adsorbent was crushed into granules in a mortar, and the pH of the solution was controlled to be slightly acidic with 0.1 mol-HNO₃
The mixed solution was stirred with a magnetic stirrer at room temperature, and 5 ml of sampling was extracted with a syringe with a microsieve at predetermined intervals. The concentrations of Cs⁺ were determined by ion chromatography, and those of Sr2+ , As3+ , Cd2+ and Pb2+ were determined by ICP emission spectrometry.
The detection of As⁵he detection of As⁵⁺ was not possible by the ICP emission method.
In addition, the initial concentration of the solution was set to 10 ppm for light elements and 50 ppm for As and heavy metal ions according to the measurement specifications of the analyzer, and the sample consumption was adjusted accordingly.
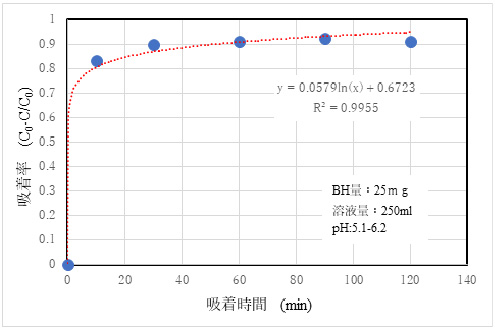
2)Adsorptivity of Cs+
Fig. 5 shows the results of adsorption experiments using a standard solution with 10 ppm CsCl.
3)Adsorptivity of Sr2+
The results of adsorption experiments using standard solutions with 10 ppm SrCl₂ are shown in Fig. 6.
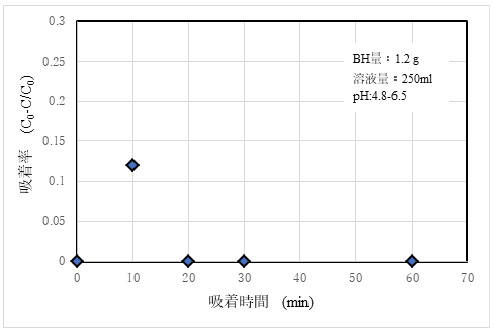
4)Adsorptivity of As3+
The results of adsorption experiments using 50 ppm As₂O₃ standard solution are shown in Fig. 7.
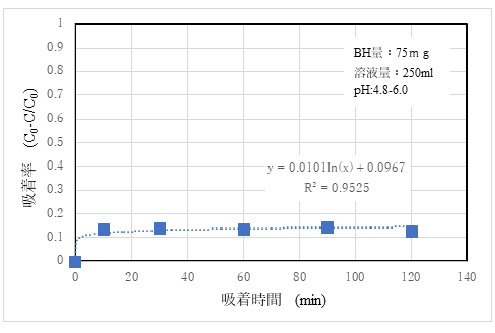
5)Adsorptivity of Cd2+
Fig. 8 shows the results of adsorption experiments of Cd₂+ using a standard solution derived from 50 ppm Cd (NO₃)₂
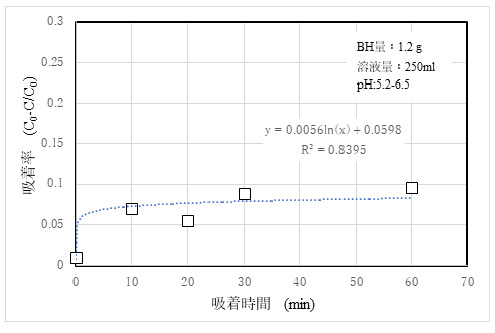
6)Adsorptivity of Pb2+
Fig. 9 shows the results of Pb2+ adsorption experiments using standard solutions from 50 ppm Pb (NO₃)₂.
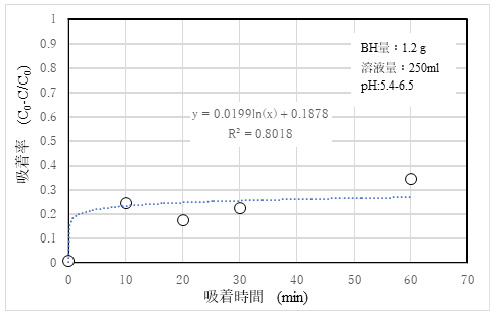
6.Consideration of Experimental Results
2)Consideration of ion adsorption performance
①This indicates that the adsorption performance of Cs and Pb is excellent in ion adsorption. The partition coefficient of Cs is estimated to be about 10⁴ (ml/g), and the adsorption amount of Pb is estimated to be about 5 times more than that of iron-based adsorbents, so it is not an exaggeration to say that this is a characteristic of BH. The pore size of Mordenite, which is a kind of zeolite used, is assumed to be about 0.6 -0.8 nm, the radius of Cs+ is assumed to be about 0.2 nm, and Pb2+ is assumed to be about 0.64 nm.
②The amounts of adsorbed Sr and Cd ions were low. This is considered to be due to the fact that the ionic radius of Sr2+ is 0.2 nm, but the hydration radius of Sr2+ is larger than 0.4 nm ²). It is explained that Cd2+ has an ionic radius of 0.8 nm, which is equivalent to or greater than that of the Mordenite pores, and that Cd2+ hardly enters the pores and has a low adsorption efficiency. However, since it is expected to be relatively inexpensive, it is inferred that it is possible to secure a practical capability by increasing the amount of use compared with the competing products.
③As (III) ion was judged to have no effective adsorption capacity. Although details are given in Report ³), As3+ is temporarily adsorbed by ion exchange with hydroxyl groups on the material surface, but it is not imparted with a permanent charge, and it is assumed that As3+ is released into the solution again by a slight dissolution action on the surface.
7.Summary
2)It can be said that BH has a excellent adsorption performance for Cs and Pb.
3)The amount of Sr and Cd used should be increased to ensure their practicability.
4) The adsorption ability for As (III) was not demonstrated, and improvement is necessary.
<Other supplementary notes>
This study was conducted by the Shobara Factory of Syoken-Techno Co., Ltd. and the Osaka Laboratory of Techno Solute Lab Co., Ltd. conducted experiments, analyses, and evaluations.
The Nakadaira Laboratory of the Engineering Department of Osaka Prefectural University and Takasago Industry Co., Ltd. cooperated in the experiment.
